Part A Answer
The first five ionization energies (IE_1 through iE_5) of a Period 2 element have the following pattern: Make a reasonable guess about which element this is. Enter its chemical symbol below.
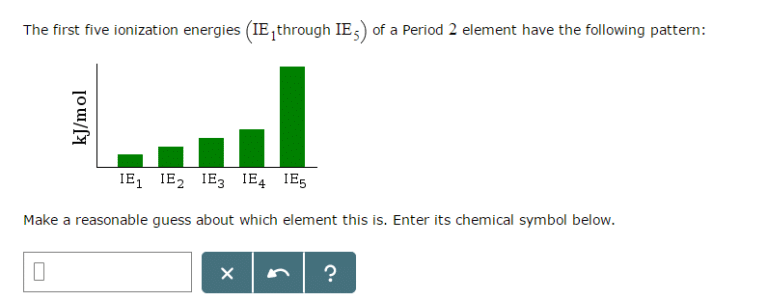
There is a huge difference in ionization energy between IE4 and IE5.
This means lots of energy are required to remove the 5th electron.
So, after 4 electrons are removed, the element has acquired a noble gas configuration,.
So, either are 4 valence electrons in the element.
The element in period 2 with 4 valence electrons is Carbon.
Answer: C
Part B Answer
The first five ionization energies (IE_1 through iE_5) of a Period 3 element have the following pattern: Make a reasonable guess about which element this is. Enter its chemical symbol below.
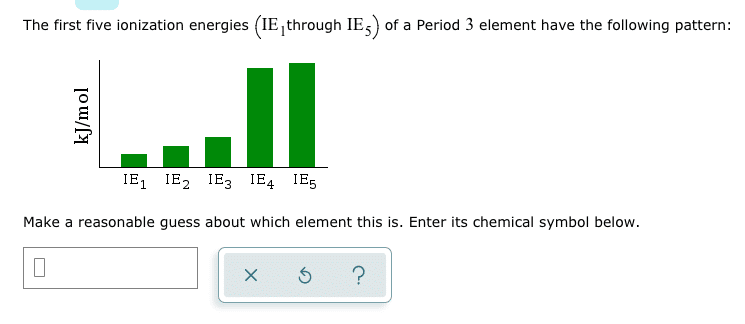
We know that Group 3 has the following elements :
Na, Mg, Al, Si, P, S, Cl, Ar.
We will approach towards the solution by considering their electronic configuration.
Now , we get two elements which Ioncan haveisation Energies as per our requirement.
But In the case of P IE1 , IE2 and IE3 are almost comparable and IE4 is not very much greater than IE3. However, in our question, IE4 has to be considerably greater than IE3. So, P cannot be our element.
In case of Al , IE1<IE2<IE3<<IE4<IE5
So Aluminium is our required element.



