Different variations of this question are answered on this page.
When a honeybee flies through the air it develops a charge of +20pC. How many electrons did it lose in the process of acquiring this charge?
Express answer as a number.
Total charge acquired= +20 pC = 20*10^-12 C
Since the honeybee was neutral before, the total charge acquired would be equal to n*charge of electron, where n is number of electrons lost
charge on each electron = 1.6*10^-19 C
n*1.6*10^-19 = 20*10-12
n=12.5*107
Number of electrons lost = 12.5*10^7
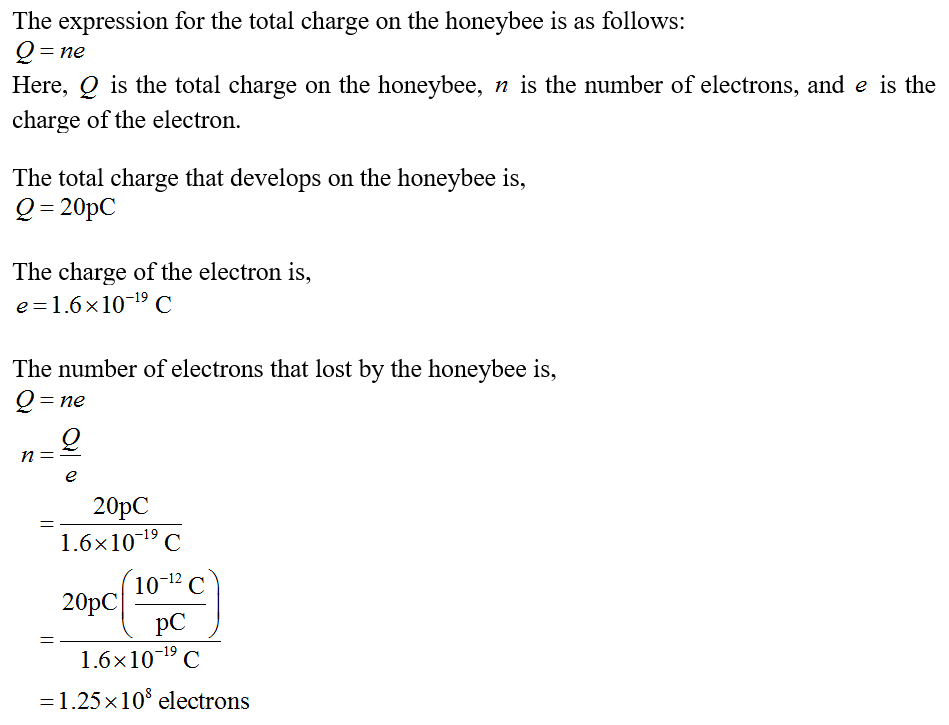
When a honeybee flies through the air it develops a charge of +19pC. How many electrons did it lose in the process of acquiring this charge?
Express answer as a number.
charge , Q = + 17 pC = 17 * 10^-12 C
magnitude of charge on electron ,q = 1.6 * 10^-19 C
the number of electron , n = Q/q
n = 17 * 10^-12 /( 1.6 * 10^-19) electrons
n = 1.06 * 10^8 electrons
When a honeybee flies through the air it develops a charge of +18pC. How many electrons did it lose in the process of acquiring this charge?
Express answer as a number.
As we know,
Charge on an electron = -1.6×10^(-19) C
According to question when a honeybee flies in the sky it developes a charge = +18pC
Which is equal to +18×10^(-12) C
We can say, when one electron is lost by any body then +1.6×10^(-19) C charge is developed on that body.
So to develope a charge of +18×10^(-12) C number of electrons to be lost by the body is = 18×10^(-12) ÷ 1.6×10^(-19) = 112500000 electrons
Therefore,
honeybee losses 112500000 (11.25×10^7) electrons to get charge of 18×10^(-12) C.
When a honeybee flies through the air it develops a charge of +17pC. How many electrons did it lose in the process of acquiring this charge?
Express answer as a number.
charge , Q = + 17 pC = 17 * 10^-12 C
magnitude of charge on electron ,q = 1.6 * 10^-19 C
the number of electron , n = Q/q
n = 17 * 10^-12 /( 1.6 * 10^-19) electrons
n = 1.06 * 10^8 electrons
When a honeybee flies through the air it develops a charge of +16pC. How many electrons did it lose in the process of acquiring this charge?
Express answer as a number.
Q=+16 pC→16×10^-12 C
n = Q/e
n = 1.0 * 10^8 electrons = 10^8.
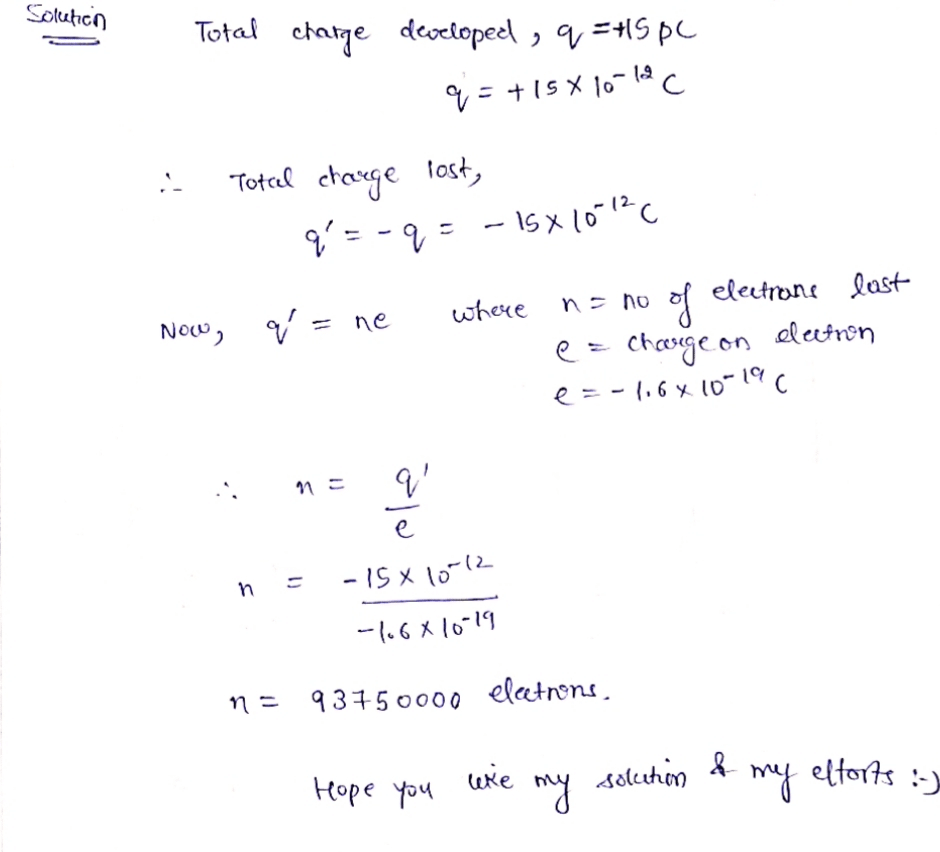
When a honeybee flies through the air it develops a charge of +15pC. How many electrons did it lose in the process of acquiring this charge?
Express answer as a number.
n = 93750000 electrons
When a honeybee flies through the air it develops a charge of +14pC. How many electrons did it lose in the process of acquiring this charge?
Express answer as a number.
n = 8×75^7 electrons




