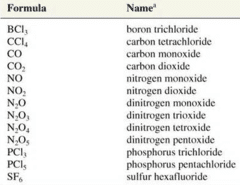
Molecular compounds are inorganic compounds that take the form of discrete molecules. Examples include such familiar substances as water (H2O)(H2O) and carbon dioxide (CO2)(CO2). These compounds are very different from ionic compounds like sodium chloride (NaCl)(NaCl). Ionic compounds are formed when metal atoms lose one or more of their electrons to nonmetal atoms. The resulting cations and anions are electrostatically attracted to each other.
So what holds the atoms of a molecule together? Rather than forming ions, the atoms of a molecule share their electrons in such a way that a bond forms between pairs of atoms. In a carbon dioxide molecule, there are two of these bonds, each occurring between the carbon atom and one of the two oxygen atoms.
Terms and Answers to learn

~Di-2
~Tri-3
~Tetra-4



