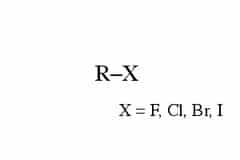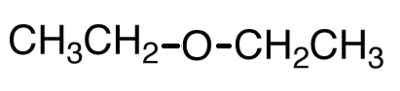Functional groups are groups of atoms that occur within organic molecules and confer specific chemical properties to those molecules. When functional groups are shown, the organic molecule is sometimes denoted as “R.” Functional groups are found along the “carbon backbone” of macromolecules which is formed by chains and/or rings of carbon atoms with the occasional substitution of an element such as nitrogen or oxygen.
Molecules with other elements in their carbon backbone are substituted hydrocarbons. Each of the four types of macromolecules—proteins, lipids, carbohydrates, and nucleic acids—has its own characteristic set of functional groups that contributes greatly to its differing chemical properties and its function in living organisms.
What are the six main functional groups?
The names of the six most important functional groups are:
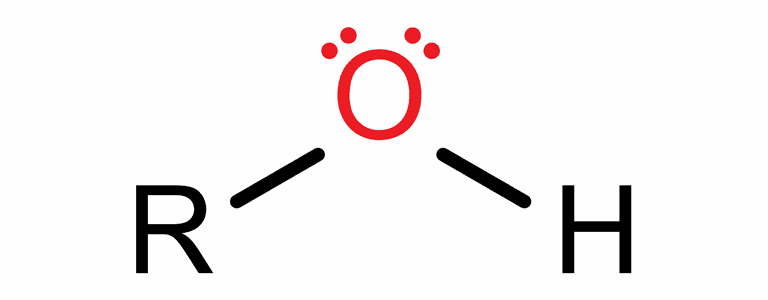
- Hydroxyl.
- Carbonyl.
- Carboxyl.
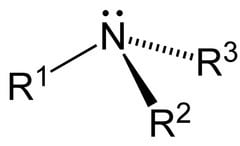
- Amino.
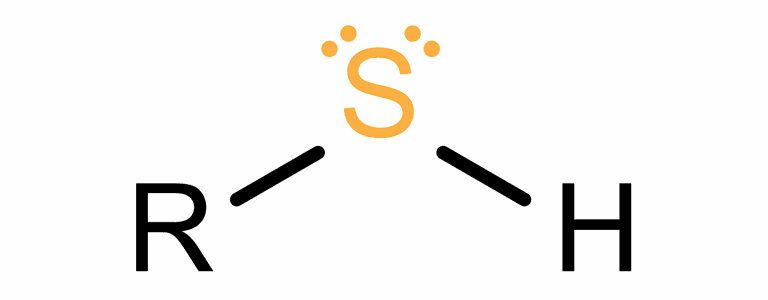
- Sulfhydryl.
- Phosphate.
Identifying Functional Groups Practice
Functional Group Practice
1. Classify the compound based on the functional group present.
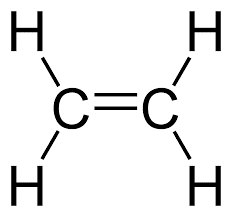
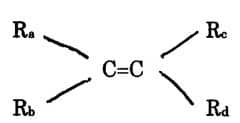
- alkane
- alkene
- alkyne
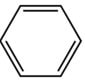
- aromatic
2. This functional group shows up in so many alcohols it has come to be known as the alcohol group. It’s really called:
- hydroxyl
- ho group
- oxyhol
- carboxyl
3. Identify the organic class to which the compound belongs.
- alcohol
- ketone
- aldehyde
- carboxylic acid
- ester
- halide
- ether
- amine
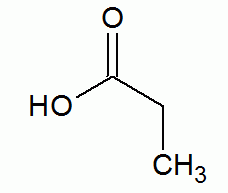
4. Classify the compound based on the functional group present.
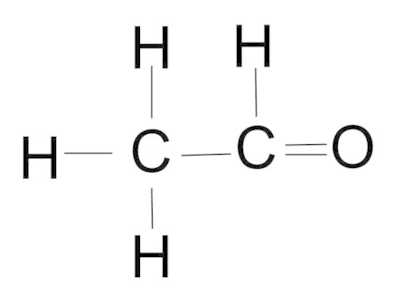
- aldehyde
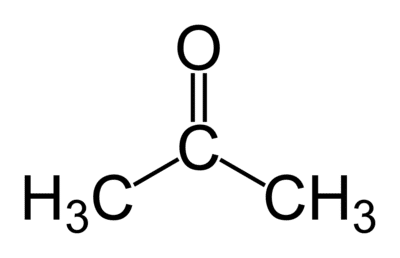
- ketone
- carboxylic acid
- ester
5. Classify the compound based on the functional group present.
- aldehyde
- ketone
- carboxylic acid
- ester
6. Classify the compound based on the functional group present.
- alcohol
- ketone
- ester
- ether
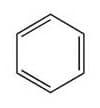
7. Classify the compound based on the functional group present.
- alkane
- alkene
- alkyne
- aromatic
8. Identify the organic class to which the compound belongs.
- aldehyde
- ester
- carboxylic acid
- ether
- ketone
- halide
- amine
- alcohol
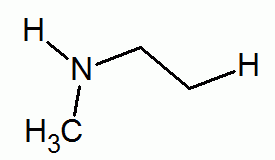
9. Identify the organic class to which the compound belongs.
- aldehyde
- amine
- ester
- alcohol
- ether
- carboxylic acid
- ketone
- halide
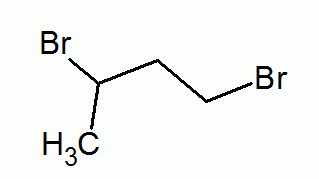
10. Classify the compound based on the functional group present.
- alkane
- alkene
- alkyne
- aromatic
11. Replace the oxygen of the hydroxyl group with sulfur and you get this functional group.
- sulfoxyl
- sulfa
- librarian (-SH)
- thiol
12. Classify the compound based on the functional group present.
- amine
- carboxylic acid
- alcohol
- ester
- ketone
- aldehyde
- halide
- ether
13. Classify the compound based on the functional group present.
- alkane
- alkene
- alkyne
- aromatic
14. Classify the compound based on the functional group present.
- ether
- aldehyde
- ketone
- alcohol
- amine
- carboxylic acid
- ester
- halide
15. Classify the compound based on the functional group present.
- carboxylic acid
- alcohol
- ketone
- aldehyde
- ester
- amine
- ether
- halide
Functional Groups Study: Terms to Learn
R-H
(CH₃CH₃)
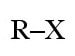
(halo group)
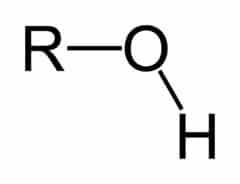
R-OH
functional group: -OH
(hydroxy group)
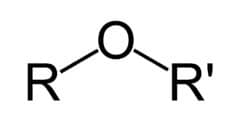
R-O-R
functional group: -OR
(alkoxy group)
R₂NH (secondary amine)
R₃N (tertiary amine)functional group: -NH₂
(amino group)
R-SH
functional group: -SH
(mercapto group)
R-S-R
functional group: -SR
(alkythio group)
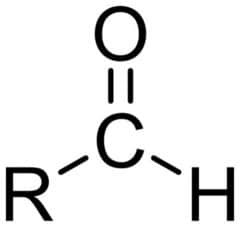
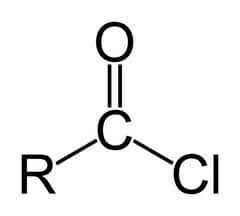
(carbonyl group)
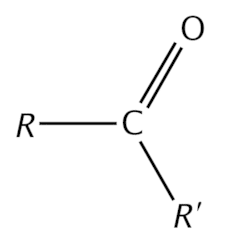
(carbonyl group)
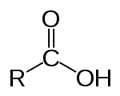
(carboxy group)
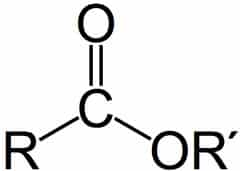
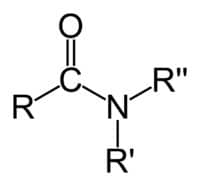
-CONHR (secondary amide)
-CONR₂ (tertiary amide)