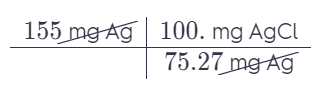Silver chloride, often used in silver plating, contains 75.27% Ag by mass. Calculate the mass of silver chloride required to plate 155 mg of pure silver.
Answer: = 205,93 mg AgCl
Each percentage represents the number of milligrams of an element per 100 milligrams of that compound. Use this as a conversion factor.