n atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, using the notation explained below.
Electronic configurations describe each electron as moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions. – wikipedia
Electron Configuration Quiz
Electron Configuration – Basic Introduction
Test Questions and Answers
1. What atom matches this electron configuration? 1s22s22p63s2
- Neon
- Magnesium
- Aluminum
- Potassium
2. What atom matches this electron configuration? 1s22s22p63s23p64s23d10
- Zinc
- Copper
- Nickel
- Germanium
3. What is the electron configuration for a Sulfur atom?
- 1s22s22p63p6
- 1s22s22p63s23p6
- 1s22s22p63s23p4
- 3p4
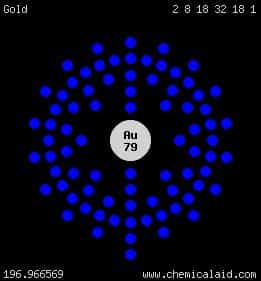
4. What atom matches this electron configuration? 1s22s22p63s33p64s23d104p65s24d105p66s24f145d9
- Mercury
- Gold
- Platinum
- Thallium
5. What electron configuration matches an oxygen atom?
- 1s22s22p63s2, 3p64s23d104p5
- 1s22s22p4
- 1s22s22p6
- 1s22s22p63s23p64s23d1
6. Which of the following is the smallest in size?
- N
- S
- I
- Fr
7. Which of the following is the smallest in size?
- K
- Na
- Li
- Cs
8. How many electrons does Si contain?
- 14
- 28
- 2
- 4
9. How many valence electrons does Si contain?
- 14
- 28
- 2
- 4
10. How many electrons can the first energy level hold?
- 1
- 2
- 8
- 0
11. An orbital can at most hold how many electrons?
- 1 electron
- 2 electrons
- 3 electrons
- 4 electrons
12. The electron configuration of an atom is 1s22s22p6. The number of electrons in the atom is
- 3
- 6
- 8
- 10
13. The electron configuration of an atom is 1s22s22p6. The number of valence electrons in the atom is
- 3
- 6
- 8
- 10
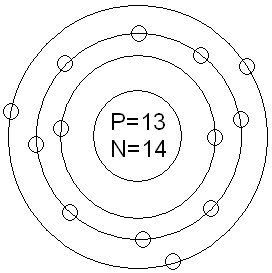
14. What atom is represented here?
- Carbon
- Nitrogen
- Oxygen
- Fluorine
15. How many valence electrons are represented here?
- 7
- 5
- 2
- 8
16. What is the electron configuration for this atom?
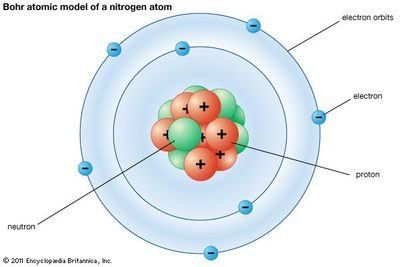
- 1s22s22p6
- 1s22s22p5
- 1s22s22p3
- 2s22p3
17. What is the highest occupied energy level?
- 1
- 2
- 3
- 4
18. What is the highest occupied energy level?
- 4
- 5
- 6
- 7
19. How many valence electrons are there?
- 1
- 2
- 8
- 79
20. How many orbitals are in the 4s sublevel?
- 1
- 2
- 3
- 4
21. How many orbitals are in the 4p sublevel?
- 2
- 3
- 4
- 6
22. How many orbitals are in the 4d sublevel?
- 2
- 4
- 5
- 10
23. How many orbitals are in the 4f sublevel?
- 2
- 4
- 7
- 14
24. After filling 5s, electrons would fill…
- 5p
- 4d
- 5d
- 3f
25. After filling 6s, electrons would fill
- 4f
- 5d
- 6p
- 7g
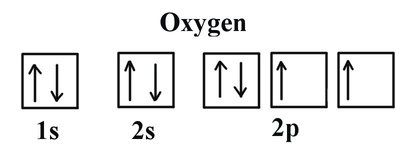
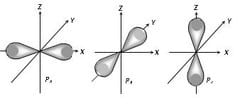
26. How many unshared pairs of electrons are in this orbital diagram?
- 2
- 3
- 5
- 15
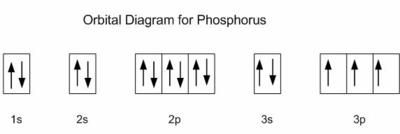
27. How many unshared pairs of electrons are in this orbital diagram?
- 2
- 4
- 6
- 8
Term and Answer Keys
Great Sources:
- https://en.wikipedia.org/wiki/Electron_configuration
- Electron configuration at LibreTexts Chemistry
- Electron configuration at FSU Chemistry
- How to Write Electron Configurations for Atoms of Any Element at WikiHow