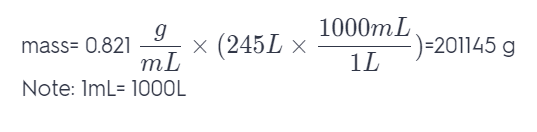A small airplane takes on 245 L of fuel. If the density of the fuel is 0.821 g/mL, what mass of fuel has the airplane taken on?
Answer: 201145g.
Explanation
To compute the mass of the fuel. Use the formula mass = density x volume. And since we are given the value of density, we just substitute it to the formula since the density of a compound is constant at a given temperature. So we have: